BASIC METALLURGY
INTRODUCTION
The basic metallurgy module will review some basic metallurgical facts and metal shaping processes that determine the physical structure of components. With this knowledge, it is possible to recognize flaws that result from material refining and metal processing operations. The module "Principles of Fractures, shows that parts fail in specific locations when they are overloaded. If a part fails anywhere else than at those locations, a material or process flaw may be present in the part.
METALLURGY
Metallurgy can be defined as the science of metals and alloys devoted to the study of engineering materials. This module covers some of the metallurgy of metal refining, metal forming, and heat treatment.Also included in this module is a discussion of some material and process flaws that can result from these operations. Manufacturing operations employ various non-destructive testing (NDT) methods to detect material and process flaws. Some of these NDT tests include hardness testing, radiography and ultrasonic testing.
The metallurgical principles contained in this section have been simplified to help students understand general concepts.
REFINING OF METALS
Cast iron and steel are the two most common metals used in Caterpillar products. Cast iron and steel are produced from iron ore that is found in deposits that consist of stable iron oxide mixed with impurities.Iron ore is refined by heating it with coke (coal baked without oxygen) and limestone in a blast furnace lined with refractory brick. As these three materials melt, two important things happen: (1) coke combines with oxygen from the iron ore releasing molten iron which sinks to the bottom of the furnace, and (2) limestone combines with impurities (dirt, sulfur, etc.) and floats to the top forming slag. These chemical and physical actions produce a less stable, refined metal called pig iron that is tapped off for further refinement into steel or cast into ingots called pigs.
Electric arc furnace operation
Steel is pig iron that has gone through a refining process to lower the carbon content, further reduce impurities, and to adjust percentages of other elements. Today, most steel is produced in electric arc furnaces. Pig iron is charged into the brick lined furnace, along with scrap, and carbon electrodes are lowered to within a few inches of the metal. The current is turned on and heat from the metal's resistance to current flow quickly melts the charge. Refining continues until the levels of carbon, impurities, and certain other elements reach required specifications. Since these processes are imperfect, some small particles of furnace brick and slag, as well as some dirt from the ore still remain in the steel.
Steel ingot structure
The fully refined steel, called a heat, is poured into ingot molds and allowed to solidify. While the steel is cooling some trapped gas may form internal voids in the ingot. Ingots are later reheated and taken to a rolling mill for hot rolling into sheets, slabs, plates or bars. During rolling most of the internal voids weld together
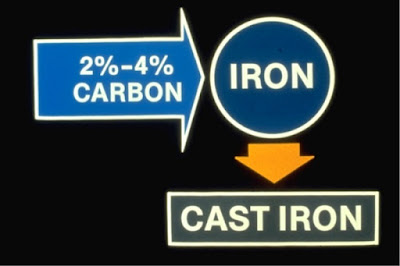
Cast iron" is iron containing 2-4% carbon
The percentage of the element "carbon" together with the element "iron" determines whether a metal is identified as cast iron or steel, and also determines most of the cast iron or steel's properties. Most cast iron is refined until it contains between 2% and 4% carbon making it brittle with little or no ductility.
Steel" is iron with less than 2% carbon
When carbon levels in iron are adjusted from .02% to 2.0%, steel is produced. Steel is generally stronger, harder, tougher, more ductile and shock resistant than cast iron.
Steels are classified by carbon content
Steels are classified according to their carbon content.Low carbon steels contain from .02% to .25% carbon, are very ductile, and are used to make non-heat treated parts such as oil pans and fuel lines. Some low carbon steels have additional carbon added to the surface (called carburizing) so they can be used for making case hardened parts such as gears and piston pins. This will be discussed in more detail later in this section.
Medium carbon steels contain from .25% to .50% carbon, are less ductile, can be heat treated and are used to make many forged parts such as crankshafts and connecting rods.
High carbon steels contain .50% to 2.0% carbon, are brittle, and are used to make fuel injection pump components and springs.
Alloying can improve steel properties.
Any of these steels can be alloyed with other metals and elements to improve physical properties such as tensile strength, toughness and corrosion resistance.Common alloying agents are chrome, nickel, vanadium, tungsten and molybdenum. These modified steels are called alloy steels.
METAL STRUCTURE
A basic principle of metallurgy is that the structure of a material determines its properties. Since the properties of cast iron and steel are so different, it is reasonable that there is probably a difference in the structure of these two very different metals. Before looking at each type of metal individually, it would be useful to look first at the general physical structure of metals.All metals are composed of grains that are crystals that have grown from the molten metal as it solidified. Grains in metal can be compared with ice crystals that form on windowpanes on a frosty morning. When the growing crystals bump into each other, they form random grain boundaries.
In cast irons and steel, the grain boundaries usually contain impurities such as refractory brick or trapped slag left from the refining process. These impurities are called inclusions and are usually small enough not to weaken the metal. In fact inclusions are seldom harmful unless they are too big or in the wrong place - such as a high stress area of a part
Cast iron structure
In this illustration of the grain structure of cast iron, notice that the grain boundaries produce a random grain structure. In addition, each grain contains carbon in the form of graphite flakes. This free carbon produces most of the properties of cast irons such as brittleness, good wear resistance, good machinability, etc.Most people use the generic term "cast iron" when they actually mean gray cast iron - the material illustrated here. Gray cast iron is used for many different parts including diesel engine heads, blocks and liners; covers; etc.
Other types of cast iron are produced by changing the shape of the graphite flakes or the structure of the metal grains.
Steel structure.
In this illustration of the grain structure of steel notice that there are no graphite flakes, but only grains and grain boundaries. The lower carbon content of steel allows carbon to mix in the grains and no free carbon exists to form flakes.This structural difference between cast iron and steel is what produces the excellent properties of ductility, strength and toughness that are associated with steel. (Note: Inclusions have been left out of the drawing for simplicity.).
Metal grains are composed of unit cells
A close look at the grain structure of a low carbon steel material reveals an orderly arrangement of "unit cells" in rows and columns much like a stack of bricks. The concept of unit cells was developed to understand how atoms are arranged in a crystal structure in metals.Notice that the alignment of the rows and columns is different in each grain. This will be important in trying to understand loading and steel failure.
The different alignment of the rows and columns in metal grains helps to explain crack growth and appearance.
Unit cells are the smallest building blocks of grains
Unit cells are the smallest building blocks of a grain.In cast iron and steel, these cells are usually cube shaped with iron atoms at the cube center and corners (called a body centered cubic structure).
The atoms in a unit cell can be rearranged or different atoms can be added by heat treating and alloying.
This gives metals different physical and chemical properties.
Forming processes
METAL FORMINGMETHODSBoth iron and steel can be made into useful forms using the metallurgical forming processes of casting and shaping. Casting processes are used to form most iron parts used in Caterpillar product. Steel parts used in Caterpillar products are formed by casting processes or by using one of the following high temperature, high pressure shaping processes:
1. Rolling
2. Forging
3. Extruding
4. Drawing
Steel that is reshaped by one of these processes is referred to as "wrought".
Cast and wrought materials form two general categories of metal with very different properties.
Casting
The casting process consists of pouring iron into a mold containing the desired part shape. Pressing a pattern of the part into sand mixed with a binder (glue) produces these molds. During the molding process, the sand sets up and holds the shape of the pattern. Internal cavities are produced by core molds supported on small iron pins called chaplets. Hot air and gasses contained in the sand and molten metal escape through small passageways in the mold called vents. Molten iron at a temperature of 1200-1315 oC (2200 - 2400 oF) is poured into the mold, flows into the mold passages, melts chaplets, produces gasses which are vented out of the mold and then begins to solidify
Castings may contain flaws
The casting may contain some trapped gas or slag inclusions, or if not properly designed for slow cooling, it may contract too quickly and pull away from itself forming shrinkage cavities.The solidified casting consists of a random grain structure with graphite flakes. No further forming can be done to this brittle structure, but it can be easily machined and heat treated.
Notice that the "structure" of the "casting" in the illustration has a mottled, uniform appearance. This is deliberate and indicates that the properties of the material are uniform. In other words, material properties from a test specimen taken in a vertical direction would be about the same as material properties from a test specimen taken in the horizontal direction. This is characteristic of cast materials.
Rolling
Rolling is the most common process used to produce wrought steel shapes such as sheets, slabs, plates, and bars. Rolling is usually done with steel heated to about 1200 oC (2200 oF) to make it very plastic. High pressure is applied with large steel rollers and the steel is squeezed into the desired shape. These shapes can then be reformed later by forging, extruding, drawing, or using other metal working processes.
Effect of rolling
During rolling, the rows and columns of unit cells in the grain slip past each other allowing the grain boundaries to take on a new shape without tearing apart. The new grain structure along with the inclusions aligns in the rolling direction forming grain flow lines. This makes the steel stronger in the direction of the grain than across it. However, cracks will grow faster with the grain just like wood splits easier with the grain.So, it is a characteristic of worked, or shaped, metals that material properties will be different in different directions. This is a result of the grain flow in the part.
Grain flow can also affect the appearance of a fracture surface when the fracture follows the grain flow in a part. The analyst should be careful not to blame a fracture on a naterial problem when the fracture surface looks different because it has followed the grain flow in the part.
Forging
Forging is a common method of forming structural shapes by heating to about 1200 oC (2200 oF) and applying force with a forging hammer or mechanical press.The hot steel grains are forced into the shape of the forging die causing grain flow to be parallel to maximum anticipated loads.
This operation sometimes produces folds in the metal called laps, or overheats the metal resulting in forging burns.
Cast metals
The key principles to remember about cast and wrought materials are that cast materials have a random grain structure containing graphite flakes while wrought materials have flow lines. This accounts for most of the difference in physical properties and behavior of these two materials. The difference in grain structure between cast and wrought materials also accounts for the different fracture appearance between cast and wrought metals.
HEAT TREATMENT
Once parts have been formed and machined, they may require heat treatment for increased strength, toughness or wear resistance. Heat treating changes the unit cell structure of cast iron and steel and puts carbon atoms into the cells making the cells harder. There are many different processes used to heat treat parts. One heat treating process commonly used by Caterpillar is called direct hardening or quenching and tempering. This heat treatment process consists of three steps: (1) austenitizing, (2) quenching, and (3) tempering.
Austenitizing
"Austenitizing" is the first stage of heat treatment and is done at elevated temperatures to put carbon into the unit cell. Carbon steels at room temperature have a body centered cubic unit cell which does not have room for carbon atoms in it. During austenitizing, temperatures are increased above 760 oC (1400 oF) to change the unit cell from body centered cubic to face centered cubic that has room for carbon atoms. The face centered cubic unit cell has iron atoms located at each cube corner and in the center of each cube face. This leaves room for the carbon atoms to fit on each cube edge. This ability to add carbon to the cell structure is the basis for heat treatment of metals.
Furnace heating is a common way to austenitize a par
Parts can be austenitized in several ways, the most common being furnace heating. Hardening furnaces are used to through heat parts for quenching. Here is an orange-hot crankshaft at a temperature of 870 oC (1600 oF) coming out of a hardening furnace.
Induction heating is used to austenitize the surface of a part for hardening
Some parts only require a hard surface layer and do not need to be through heated. Induction heating is a method of electrically heating part surfaces about 6 mm (.250 inches) deep. Here is a crankshaft with the surface layer being austenitized by induction heating.
Flame heating is used to austenitize the surface of a part for hardening
Flame heating is a third method of surface heat treatment used to austenitize metal surfaces about 12 mm (.5 inches) deep. Quenching and tempering immediately follows all of these methods of austenitizing.
Quenching
"Quenching" is the second stage of heat treatment consisting of rapidly cooling the metal from the austenitizing temperature to room temperature.Upon quenching, the face centered cubic (FCC) unit cell changes to a new form called body centered tetragonal (BCT) that locks carbon into it.
This produces steel of high strength and hardness that is also very brittle and contains high residual stresses. A typical medium carbon steel crankshaft will have hardness increased from Rockwell C20 to Rockwell C60 after quenching. (These hardness numbers will be explained later.).
Quenching media
Parts may be quenched in water, oil or air. Water can usually be used on low and medium carbon steels, while oil or air is used on high carbon or alloy steels.Caterpillar prefers to use water quenching whenever possible. Water is safer than oil (which is flammable) and tends to produce better mechanical properties than oil quenching.
Tempering
"Tempering" is the final stage of heat treatment and relieves residual stresses while improving toughness through controlled escape of some carbon from unit cells. Careful control of tempering temperatures gives good control of the carbon escape rate and minimizes hardness loss. Unless parts are raised above this tempering temperature, the final toughness and hardness will remain stable. Tempering of the crankshaft seen previously reduces the hardness to about Rockwell C52.
Case Hardening
Another type of heat treatment used to produce hard surface skins on parts is called "case hardening". Since medium and high carbon steels are expensive, it is less expensive to use a low carbon steel for case hardened parts and add carbon, nitrogen (another alloy element like carbon) or both to the surface layers to be hardened. Three common heat treatments to do this are carburizing, carbo-nitriding, and nitriding.Carburizing is done at a temperature of about 927 oC (1700 oF) and can add carbon to a depth of about 3 mm (.125 inches). It requires quenching and tempering afterwards and produces a hard, wear resistant surface with a tough supporting core capable of carrying high loads. Quenching from the high carburizing temperature produces a lot of distortion in carburized parts which must be removed by straightening or machining back to correct specifications.
Carbo-nitriding can add carbon and nitrogen to a depth of about .3 mm (.015 inches) at about 760 oC (1400oF), requires quenching and tempering afterwards, and produces a thin, hard, wear resistant surface. The lower temperatures for this heat treatment reduce part distortion.
Nitriding adds nitrogen to about .3 mm (.015 inches) of surface metal at about 538 oC (1000 oF), requires austenitizing, quenching and tempering before the nitriding operation and produces a hard, wear resistant case with a tough supporting core capable of carrying medium loads. The very low nitriding temperature produces no distortion and can be applied to finished parts without changing the dimensions
Hardness testing
Hardness tests are used to measure the effectiveness of heat treatment. The Rockwell C hardness test measures a metal's resistance to indentation by a diamond indenter that is pushed into the metal surface by a very accurately determined amount of weight. The harder the metal, the shallower the indentation. A typical nonheat treated machinable steel part will have a Rockwell C hardness less than Rc 20. Parts harder than Rc 40 are not machinable and must be ground. Hardened wear surfaces and heat treated cases may be Rc 45 - 65
MATERIAL AND PROCESS FLAWS
Forming processes can introduce surface and internal defects in both cast and wrought metals. It is necessary to become familiar with the appearance of surface and internal defects in order to recognize them on fracture faces.Internal defects can start internal cracks that eventually grow to the surface of a part while external defects can start cracks that grow internally in the part.
The key idea to remember is that when cracking starts internally, a flaw is usually going to be found at the starting point. The presence of such a flaw indicates faulty material, processing or perhaps severe overloading. It is now time to take a more detailed look at the most common defects learning what causes them, their location in parts, and the appearance of each.
Inclusions
Refining, shaping and heat treatment are all metallurgical processes that can leave flaws or defects in materials. Refining may not remove all the dirt from the ore, may leave slag trapped in the melt, or may introduce furnace brick particles into the metal. These trapped impurities, called defects or inclusions, are present in all materials but are usually too small to cause any problems. Again, inclusions are not usually harmful unless they are too big or in the wrong place
Inclusions (arrow)
Inclusions (arrow) are usually so small after being rolled or forged that they do not cause failures. Occasionally one may be in a critically loaded area, and may be large enough to start a crack. Since these flaws are mostly internal, cracking will start inside the part when it starts due to inclusions like dirt, slag or brick.
Casting Shrinkage
A common internal casting flaw is a shrinkage (arrow) cavity caused by pouring iron too hot or not providing enough metal to thick casting sections. As metals cool, they contract and more metal must flow into these areas to keep the casting from pulling apart. If this is not done, large irregular shaped cavities will form inside the metal weakening it in that area. If the cavity is too large, or if unusually high loads occur during operation, a crack may start at the cavity.
Handling Cracks
A common casting surface flaw is handling cracks (arrow) caused by physical abuse. When opened, they have no discoloration unless exposed to heat or corrosive environments while in service. Fatigue cracks, which will be discussed in detail in the Principles of Fracture module, can grow from these surface cracks if loads are high
Pipe Defects
The three most common internal defects in wrought materials are pipe, (arrow) flakes, and forging hot spots. Pipe occurs when steel ingots solidify leaving a shrinkage cavity in the top center portion of the ingot. This part of the ingot is usually sawed off before rolling. Occasionally, some of the cavity remains in the ingot and is rolled into the finished shape. This forms an irregular hole in the center referred to as pipe. Pipe can be a stress raiser and generate an internal fatigue crack in a part during service.
Hydrogen Flakes
Flakes (arrow) are caused by hydrogen gas being dissolved in the steel during the refining process. Hydrogen collects around inclusions, creates high pressure and bursts the steel internally. Flakes look like small, shiny, rounded bright spots when present on fracture faces.
Forging Burns
Overheating steel during forging causes forging hot spots. Heat generated from the severe mechanical working raises the grain boundary temperature in the heaviest worked areas to the melting point causing weakening of the grains internally. If this condition leads to failure, the melted areas will look like large grains or crystals on the fracture surface.
Seam and Lap defects (arrow)
Forging LapsSeams and laps (arrow) are common surface defects having a very similar appearance. Metal folding in on itself during rolling or forging operations causes them. Surface scale gets pinched into the seam or lap preventing it from welding to itself at the high hotworking temperatures. When present on fracture faces, they appear dark black and rough due to this scale.
Heat treat defects
In addition to refining and shaping flaws, heat treatment can produce defects such as quench cracks, soft spots and straightening cracks.
Quench Cracks
Quench cracks (arrow) are caused by heating parts too hot before quenching them, quenching them in water or oil that is too cool, or using water to quench a steel that should be quenched in oil. The severe thermal shock results in metal contracting too fast and cracking at the surface. Quench cracks usually occur in stress raisers already present in the part such as gear tooth fillets, keyways, splines, and thread roots. If parts are tempered above 260 oC (500 oF) in air, the quench crack may develop some discoloration.
Straightening cracks (arrow)
Heat treatment causes residual stress in parts which usually results in distortion. A common method for removing distortion is straightening in hydraulic presses. If parts are overstressed during this operation, a crack may develop at a stress raiser. These cracks are called straightening cracks, (arrow) and they can be the source of a fatigue crack during service.
NONDESTRUCTIVE TESTING
"Nondestructive testing (NDT)" is used to monitor refining, shaping and heat treating processes to assure finished materials are free of harmful flaws. NDT is also used to find defective material by 100% sorting for flaws. Commonly used methods include magnaglo, zyglo, ultrasonic and eddy current testing. Magnaglo, zyglo, and eddy current tests are used to find surface defects while ultrasonic testing is used to locate subsurface defects.
Ultrasonic NDT
Piston manufacturers make use of ultrasonic testing equipment to verify the bond quality of the cast iron ring band that carries the piston rings. Since this was begun, the number of pistons experiencing bond failures in service has dropped drastically
Ultrasonic NDT and Monitor crankshaft material quality.
Certain engine applications, such as marine, require higher than usual crankshaft quality. Ultrasonic testing of large bore crankshafts helps certify that inclusion levels in these forgings are well below the size that can cause a failure.
CONCLUSION
This concludes the section on basic metallurgy. Metal refining, shaping, and heat treating can create flaws or defects in parts. By knowing what these flaws look like, it is possible to quickly determine if any are present on a fracture. If none are found, do not blame the part or process for the failure. Look for other facts or road signs that will help to determine the root cause of failure.Understanding basic metallurgical principles can help the failure analyst find the root cause of a problem and determine the proper corrective action. The metallurgy of Caterpillar parts is very carefully designed and closely controlled. It is important to have good supporting facts before accusing a part of causing a failure. Frequently, careful failure analysis reveals that a part has failed due to an abnormal operating condition rather than a problem with the part material or the manufacturing processes used to make the part.
THANK YOU HAVE VISITED ON WWW.GOSGT.COM.
BASIC METALLURGY
 Reviewed by heri
on
3:50 AM
Rating:
Reviewed by heri
on
3:50 AM
Rating:
 Reviewed by heri
on
3:50 AM
Rating:
Reviewed by heri
on
3:50 AM
Rating:




























































No comments: